Abstract: Of all of the butterflies found in the British Isles, the complete life cycle of the Chequered Skipper is one of the most rarely observed, for several reasons. The first is that the distribution of the butterfly is restricted to north west Scotland where the level of recording is relatively low. The second is that, while many enthusiasts have made pilgrimages to see the adult butterfly, very few have put the same effort into locating the immature stages. Finally, the ecology of the butterfly requires the observer to put in a significant amount of time before they are rewarded with views of all stages. In this article, the author summarises his observations over a three year period, from 2014 to 2016.
My first encounter with a Chequered Skipper (Carterocephalus palaemon) was on 2nd June 2006, when I visited Glasdrum National Nature Reserve (NNR), which is situated on the shores of Loch Creran in Argyllshire, Scotland. Despite the less-than-ideal weather, I managed to find two male butterflies roosting, closed-winged, among the raindrops. A return visit, two days later, proved to be much more fruitful and gave me my first opportunity to study the butterflies in more detail, in terms of both their appearance and behaviour. I have visited the area almost every year since in order to observe the specialties (of butterfly species and subspecies) found in this beautiful part of the world.
 |  |
| Figure 1 - Male Chequered Skipper (Glasdrum NNR, 4th June 2006) Image © Peter Eeles | Figure 2 - Male Chequered Skipper (Glasdrum NNR, 2nd June 2006) Image © Peter Eeles |
With a deep interest in the immature stages of the butterflies of the British Isles, I made a concerted effort in 2014 to locate those of the Chequered Skipper and, although I was only partially successful that year, was sufficiently encouraged to continue my studies through 2015 and 2016. My observations, from 2014 to 2016, form the basis of this article.
Of the many publications consulted in preparing for this study, Neil Ravenscroft's PhD thesis on "The Ecology and Conservation of the Chequered Skipper Butterfly Carterocephalus palaemon (Pallas)" (Ravenscroft, 1992) was a particular inspiration. This work went on to inform a number of other publications including Ravenscroft (1994a, 1994b, 1994c), the Species Action Plan (Ravenscroft and Warren, 1996) and a modest Butterfly Conservation booklet (Ravenscroft, 1996). In several respects, my study was attempting to validate some of Ravenscroft's conclusions, illustrate some of his findings with photographic evidence and also build upon his work by filling in certain gaps.
The English population of Chequered Skipper became extinct in 1976 and so this article is concerned only with the Scottish population which was discovered as recently as June 1939 at Loch Lochy (Evans, 1949). Individuals from the English population had minor differences in appearance and also some behavioural differences (according to Collier (1986), they were considered more sedentary, for example).
In order to maximise my chances of finding all stages of this butterfly, I invested time in understanding its phenology (the timing of lifecycle stages), habitat requirements, population dynamics and preferred sites. Each of these topics is discussed below, followed by observations of each of the stages, an analysis of data captured and some concluding remarks.
A rudimentary phenology chart of the Chequered Skipper in Scotland is shown in Figure 1 (derived from UK Butterflies, 2014), an understanding of which helped with the planning of trips to find and monitor each stage of the lifecycle. One of the main objectives of the study was to produce a more detailed phenology chart that reflected not only each stage, but also each larval instar.
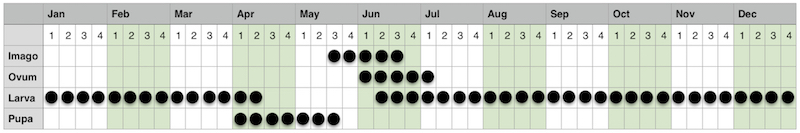 |
| Figure 3 - Chequered Skipper phenology chart Image © Peter Eeles |
The Chequered Skipper is single brooded and is typically on the wing for a relatively short period from the 3rd week of May until the 3rd week of June, although this varies somewhat from year to year and site to site. Collier (1986) gives an extreme example for 1984 when the flight season started incredibly early on 4th May and finished on 5th June, and so anyone looking to see the adult butterfly would do well to monitor relevant sources of sightings and be flexible in their plans. As far as variability between sites is concerned, Ravenscroft (1992) states that the emergence at Glasdrum NNR (one of the most southerly sites) is always a week to 10 days ahead of Ariundle NNR (one of the most westerly sites). At all sites, males emerge several days before females.
With regard to the immature stages, eggs hatch after approximately 10 days. There are 5 larval instars, the larva overwintering in the final instar when fully-grown toward the end of October, re-emerging in the middle of March and, without further feeding, pupating in early April, remaining as a pupa for around 6 weeks.
It was necessary to identify the right habitat for the Chequered Skipper in order to narrow down the search areas, and therefore reduce survey time, and this is where Ravenscroft's work really came into its own and the description below is derived, in part, from his work.
Sites are typically located at the edge of woodland in areas of scrub, but the butterfly can use other areas such as wet meadows and is not, therefore, entirely a woodland butterfly in Scotland. Whatever the site, there are some obvious and not-so-obvious requirements of adults and immature stages if the site is to play host to the Chequered Skipper and these are discussed below.
Ravenscroft (1992) suggests an "ideal" site for the adult Chequered Skipper: "open woodland, usually dominated by [Sessile] Oak Quercus petraea or [Downy] Birch Betula pubescens, on gently sloping hillsides, often by the side of lochs, in sheltered clearings that catch the sun". Ravenscroft (1992) contains a diagram of a typical site at a loch side which is reproduced and added to in Ravenscroft (1996) where a typical situation at a woodland edge is also included. These are shown in Figures 4 and 5, which I found particularly useful when visiting sites.
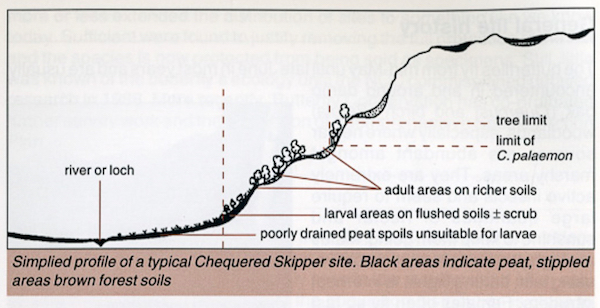 |
| Figure 4 - Profile of typical loch side habitat (from Ravenscroft, 1996) Image © Butterfly Conservation |
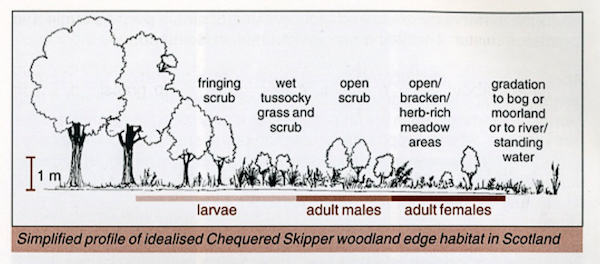 |
| Figure 5 - Profile of typical woodland edge habitat (from Ravenscroft, 1996) Image © Butterfly Conservation |
The first habitat requirement is that the site provides areas that are sheltered, allowing adults to raise their body temperature in order to maintain the level of activity that they exhibit, such as males defending their territories. This could be a bay at a woodland edge or an area surrounded by scrub, for example.
 |  |
| Figure 6 - Woodland edge habitat (Ariundle NNR, 6th June 2016) Image © Peter Eeles | Figure 7 - Scrub habitat (Ariundle, 7th June 2016) Image © Peter Eeles |
The second requirement is that the site is rich in appropriate nectar sources, given that both sexes are very active and need to constantly top up their energy reserves. Also, this food supply is almost certainly an important factor in lifespan, fecundity (number of eggs laid) and the development of eggs in the female. Ravenscroft (1992) also suggests that, based on work on the Essex Skipper by Pivnick and McNeil (1985), females may have few mature eggs when they emerge and that nectaring is therefore particularly important. He also says that individual females had been observed feeding for over 3 hours on occasion, emphasising this importance. Ravenscroft (1994a) suggests that adult resources (such as nectar sources) may be the limiting factor in terms of population distribution given that, by his estimate, the area available for larvae was three times that of adults at his study site at Ariundle.
Both sexes feed avidly on the flowers of various species (Collier (1986) lists 18 species), especially those with blue or purple flowers, Bluebell (Hyacinthoides non-scriptus), Bugle (Ajuga reptans) and Marsh Thistle (Cirsium palustre) being particular favourites, when available.
| Video 1 - Male Chequered Skipper nectaring on Bluebell (Glasdrum NNR, 12th June 2015) Video © Pete Eeles |
| Video 2 - Male Chequered Skipper nectaring on Heath Spotted Orchid (Glasdrum NNR, 27th May 2014) Video © Pete Eeles |
Suitable habitat must also provide places where the male butterfly can set up a territory (that allows him to perch and intercept passing females). Ravenscroft (1994a) states a number of characteristics of a territory.
 |
| Figure 8 - Male Chequered Skipper perching in his territory (Glasdrum NNR, 4th June 2006) Image © Peter Eeles |
The first is that the territory should offer shelter and, as discussed earlier, could be a bay at a woodland edge or an area surrounded by scrub. Ravenscroft (1996) is more specific and says that territories are more or less semi-circular in shape with a narrow opening, although the size can vary from 5m to 50m across. The second characteristic is that the territory provides good visibility; territories usually have well-spaced perches that are above the level of underlying vegetation, providing good visibility of passing insects. The third is that the territory promotes high temperatures. To achieve the high temperature required, territories are usually south facing and have relatively dry and sparse vegetation that fosters higher temperatures than vegetated areas.
In addition to providing shelter, good visibility and high temperatures, Ravenscroft (1996) also notes that territories are usually close to nectar sources, but not so close that they interfere with the purpose of a territory by attracting other males, other insects and females looking only to nectar.
Ravenscroft (1994c) poses a very good question: "Why have Chequered Skippers adopted a system where males defend territories in areas that have no resources attractive to females, despite concentrations of females occurring elsewhere [such as at nectar sources]?". He gives two possible reasons.
Firstly, from the perspective of the female, she is not looking to encounter a male unless looking for a mate. For example, while there is no conflict when the two sexes nectar together (since they are seemingly focused on feeding and oblivious to one another's presence), it has been shown that interference from other individuals will drive a female away from an area when egg-laying.
Secondly, from the perspective of the male, while he could wait for a female at a nectar source, she may go unnoticed when he is also aware of all of the other insect life that may be present at the same resource. He could also wait near larval sites but, since eggs are deposited at low density, this would not be an effective mate-locating strategy.
Ravenscroft (1992) notes similarities between a Chequered Skipper territory and a lek, used by species such as Black Grouse (Tetrao tetrix), in that there is no competition for resources used by females in these areas such as nectar sources. If a female enters such areas then she is quite possibly looking for a mate.
Given that a proportion of eggs is laid within the site itself, then it goes without saying that the site should also contain the larval foodplant which, in Scotland, is almost exclusively Purple Moor-grass (Molinea caerulea), although Collier (1986) found eggs at Loch Arkaig on False Brome (Brachypodium sylvaticum), the foodplant believed to be used by the former English population.
 |
| Figure 9 - A tussock of Purple Moor-grass (Glasdrum NNR, 1st September 2014) Image © Peter Eeles |
One of Ravenscroft's key findings (Ravenscroft, 1992) is with regard to the larval foodplant: given that the larva feeds for over 4 months before entering hibernation, it needs foodplant that remains in good condition until it has finished feeding. The vast majority of Purple Moor-grass, a widespread and abundant plant in north west Scotland, does not meet this criterion, becoming unsuitable before the larvae have finished feeding resulting in their slow development and ultimate demise. According to Ravenscroft (1994b) the difference between suitable and unsuitable plants is particularly pronounced towards the end of the larval season.
 |
| Figure 10 - The Purple Moor-grass at the bottom of this slope is greener (and more suitable) than that at the top of the slope (Glasdrum NNR, 1st September 2014) Image © Peter Eeles |
Ravenscroft (1992) puts the longevity and quality of suitable Purple Moor-grass down to several factors. These include a good water supply and a soil that is well aerated, high in nutrients and of a neutral pH of around 5-6 (it is neither too acidic nor too alkaline). In such preferred conditions, leaf blades are longer and wider. Ravenscroft (1994b) also says that the best larval survival rates were on plants that had fewer or no flowers since the period after flowering results in a decline in nutrients in the leaves.
These conditions give rise to a number of other plants, such as Bog Myrtle (Myrica gale), birch, alder and sallow, which provide an indication that the nearby Purple Moor-grass is suitable for larval development. Consequently, suitable sites for oviposition are in damp, scrubby and overgrown areas and, based on observations, are often in partial shade at the edge of a clearing or under light woodland. Conversely, although Purple Moor-grass may grow profusely in other areas, alongside plants such as Cross-leaved Heath (Erica tetralix), Common Heather (Calluna vulgaris) or rushes (Juncus spp.), they are avoided by egg-laying females.
Ravenscroft (1992) provides some specifics, and says that most success with regard to larval development at Ariundle were areas that had Bog Myrtle, Downy Birch (Betula pubescens) or Bracken (Pteridium aquilinum) present, all of which enrich the soil, with the usual combinations being all three, Bog Myrtle and Downy Birch, or Downy Birch and Bracken. Knowing that these combinations play host to Chequered Skipper larvae provided some of the best visual clues when searching out suitable areas.
Ravenscroft (1995) says that the Chequered Skipper benefits from regimes where there is browsing (feeding on the leaves and shoots of shrubs) but only limited grazing (feeding on grass or other low vegetation).
Browsing is important since it prevents succession in larval areas and also prevents scrub invasion in areas where the adults fly (such as in male territories and around nectar sources). Roe Deer (Capreolus capreolus) are primarily browsers and are present at most sites.
Grazing, however, can have a detrimental effect during the larval stage, since larvae feed quite high on the foodplant and are obviously prone to the feeding habits of Red Deer (Cervus elaphus) (although they graze woodlands mainly in the winter) and also Roe Deer (who feed on grasses in the spring).
According to Ravenscroft (1992), most species that have precise habitat requirements form close-knit colonies, such as Silver-studded Blue (Plebejus argus) and Heath Fritillary (Melitaea athalia). The Chequered Skipper, however, is not considered colonial, occurring in low densities in an open population structure and will freely disperse in order to satisfy its needs, such as finding nectar. The precise boundaries of a given population are, therefore, difficult (if not impossible) to define. The implication is that certain sites may be reused year after year not because of any colonial properties, but because the conditions are right and adults migrate into the area each year.
Based on a mark-release-recapture exercise, Ravenscroft (1992) showed that male Chequered Skippers remain relatively immobile once they have moved from their eclosion site to a favoured area, whereas females become more mobile and disperse once they have mated (and presumably lay wherever conditions are deemed favourable). When describing the distances travelled by the more-mobile female, Ravenscroft (1992) says "Just flying from the eclosion site to nectar supplies, then to a male and back to egg laying areas may take a female > 1km. in Ariundle". Ravenscroft therefore concludes that populations are dynamic and that this behaviour makes the butterfly a good colonist of new areas.
Ravenscroft (1995) sums things up rather nicely: "Carterocephalus palaemon is therefore a species that moves about freely and is one that occurs at low density throughout its range, with concentrations of adults in favoured areas".
Based on my own observations at both Ariundle NNR and Glasdrum NNR (both sites are discussed later), conditions that meet almost all of the needs of the butterfly may also exist in the core flight areas. For example, there is one area at Glasdrum that is roughly 2m by 2m in which I have found both males and females nectaring, a mating pair, 2 eggs (one of which was followed through to pupation) and 3 final instar larvae. Clearly, this particular spot is rather special since it would seem to satisfy most of the needs of the butterfly within a very small area indeed.
Ravenscroft (1992) also makes observations regarding the periods of activity and found that males are active throughout the day, with peaks late morning and early afternoon, and that females were usually seen earlier in the day, less obvious in the middle of the day, but active later in the afternoon. There appeared to be a drop in butterfly numbers in the middle of the day.
In addition to having a grasp of the phenology, habitat requirements and population dynamics of the Chequered Skipper, there was also the matter of logistics. Specifically, in order to maximise study time, this meant finding suitable sites where there was the potential for finding the butterfly in all of its stages without the need for a significant amount of travel by car or large distances that needed to be covered by foot. The relative abundance and location of adult butterflies at selected sites provided initial indicators of suitable sites and areas to focus on.
The butterfly is found across the northern hemisphere, from the British Isles, across Europe and Asia, and into Japan and North America (Ravenscroft, 1995). In the British Isles, the English population of Chequered Skipper became extinct in 1976 leaving the only remaining population in north west Scotland at a series of sites centred on Fort William.
 |
| Figure 13 - Map showing Chequered Skipper distribution Image © Peter Eeles |
Although Ravenscroft (1992) considers two study sites, Ariundle NNR and Glasdrum NNR, it is the former that received most of his attention and from where most of his findings were obtained. Both were on my list of sites to consider along with five others that had proved fruitful for other observers; the Butterfly Conservation reserve at Allt Mhuic, Glen Loy, Glen Nevis, the head of Loch Etive and the area around Spean Bridge. These sites are shown on the map in Figure 14 and each is discussed below.
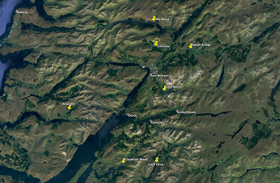 |
| Figure 14 - Map of sites visited Image © Peter Eeles |
Butterfly Conservation's reserve at Allt Mhuic lies 6 miles to the west of Clunes on the north shore of Loch Arkaig and was the most northerly site visited. The site has ample parking and a very helpful display board that shows a recommended walking route that is marked by posts containing a butterfly logo.
I made a single visit to Allt Mhuic quite late in the flight season and, while the views over Loch Arkaig were spectacular from the top of the reserve, the number of Chequered Skipper found here was relatively small when compared with other sites and this has been confirmed by other observers such as Newland (2011).
Ariundle NNR was the westernmost site visited, situated to the west of Loch Linne and the north of Loch Sunart. If coming from the east, it can be reached by driving north around Loch Linne, but a much easier and quicker alternative is to take the Corran car ferry across Loch Linne before driving west along Glen Tarbert to Strontian and then heading north to Ariundle.
There is large car park to the south-west of the reserve and Chequered Skipper were found flying either side of the path that runs more or less south-west to north-east as it leads up to and through the reserve. The car park also has an informative display board giving information about the area.
The reserve itself is part of a much broader landscape that encompasses the Ariundle Oakwoods and the land that reaches down to the Strontian River and beyond. If you like to get some exercise while out butterflying, then I recommend a visit to this part of the world; during a tour of Chequered Skipper sites in 2016 I visited Ariundle 3 days in a row and never walked less than 9 miles each day.
The entrance to the reserve itself is a little way up the path from the car park and is clearly marked with a display welcoming visitors. A somewhat-steep woodland walk to the north of the path takes you through an area that is "other worldly", with large moss-covered boulders laying among the trees. The trail eventually emerges at an old abandoned croft, complete with ever-present Small Pearl-bordered Fritillaries (Boloria selene), before winding back down to the path below. Unfortunately, the woodland walk is devoid of Chequered Skipper habitat.
Between the main path and the River Strontian are various clearings and one, in particular, had all of the hallmarks of Chequered Skipper habitat, where over a dozen Chequered Skipper was typically seen, as well as the ubiquitous Small Pearl-bordered Fritillary and other widespread species such as Green-veined White (Pieris napi ssp. thomsoni).
 |
| Figure 24 - Clearing with Chequered Skipper (Ariundle, 6th June 2016) Image © Peter Eeles |
The area the other side of the river is accessed via a path that forms a mile-long loop off and back to the main path. Coming from the south west, there is welcoming bench next to the path.
 |  |
| Figure 25 - Bench south of the River Strontian (Ariundle, 7th June 2016) Image © Peter Eeles | Figure 26 - Bench south of the River Strontian (Ariundle, 7th June 2016) Image © Peter Eeles |
This area turned out to be particularly productive, not just for the pockets of Chequered Skipper that were encountered, but also for Small Pearl-bordered Fritillary which were particularly abundant here, and the commonest butterfly species present by far, often coming down to the river to nectar on the Thyme (Thymus sp.) that grows on small sand banks.
 |  |
| Figure 27 - Area south of the River Strontian (Ariundle, 6th June 2016) Image © Peter Eeles | Figure 28 - Thyme growing on a sand bank (Ariundle, 6th June 2016) Image © Peter Eeles |
 |  |
| Figure 29 - Male Small Pearl-bordered Fritillary (Ariundle, 8th June 2016) Image © Peter Eeles | Figure 30 - Male Small Pearl-bordered Fritillary (Ariundle, 7th June 2016) Image © Peter Eeles |
Glasdrum NNR is located toward the southern end of the range of the Chequered Skipper, at the base of Beinn Churalain on the banks of Loch Creran. It is relatively easy to get to when driving up from the south and is very accessible, with its own dedicated car park and well-marked trails.
This is one of the most popular sites for visitors to see Chequered Skipper, where adult butterflies can be found in the wayleave that is regularly cleared to make way for the power lines that run through it. Compared with other sites, this area is relatively-small. Other areas within the reserve were not investigated to any great extent.
My opinion is that the area below the wayleave is not a typical Chequered Skipper site since all of the requirements of the butterfly can be found in a relatively small area when compared with other sites. Newland (2011) made a similar observation at this and other sites. This is good for those looking for both adults and immature stages (unsurprisingly, most of my observations regarding immature stages were made at Glasdrum), but not necessarily ideal for those looking to draw conclusions regarding the species ecology.
I was therefore not surprised to read that Ravenscroft (1992) chose Ariundle NNR as his main study site, rather than Glasdrum, since the extent of that reserve provides a much clearer demarcation of areas used for nectaring, mate location, egg-laying and the like (based on a number of "vegetation communities" that Ravenscroft had identified), and for any conclusions regarding the ecology of the Chequered Skipper to stand up to scrutiny.
Glen Loy lies approximately 5 miles north of Fort William and to the west of the Caledonian Canal. There is a single road through the Glen and, once you have crossed the bridge over the River Loy onto the north bank while heading west along the Glen, Chequered Skipper can be found in suitable patches between the road and the river.
Of all the sites visited, this had the highest density of midges by quite some margin and this is something to be aware of if you plan a visit.
Glen Nevis lies to the south east of Fort William, in the shadow of Ben Nevis, the highest mountain in the British Isles, at a height of 1346m above sea level. This was the least explored of the sites visited and, although no Chequered Skipper were seen during the briefest of visits, there were definitely areas that had all the markings of Chequered Skipper habitat and I was not surprised that others have found this species here.
 |
| Figure 37 - Potential Chequered Skipper habitat (Glen Nevis, 9th June 2016) Image © Peter Eeles |
Loch Etive is one of the most southerly sites for Chequered Skipper and is accessed by turning off the A82 that runs through the spectacular Glen Coe, and driving approximately 10 miles through the equally spectacular Glen Etive, where the road follows the River Etive. This really is one of the most beautiful areas to pass through, as mountains rise up from the road on either side. One noticeable characteristic of this drive during the Chequered Skipper flight period is the sheer amount of Hare's-tail Cottongrass (Eriophorum vaginatum), whose heads are unmistakable when in flower. Small Pearl-bordered Fritillary can also be found nectaring on the flowers that grow on the road verges just before the village of Gualachulain and Loch Etive is reached.
 |
| Figure 38 - Hare's-tail Cottongrass growing in profusion (Glen Etive, 9th June 2016) Image © Peter Eeles |
The end of the road signals the start of Loch Etive, where there is a small car park. A short walk along the path that runs south along the western side of the loch soon reveals suitable patches of habitat where Chequered Skipper can be found.
 |
| Figure 39 - The head of Loch Etive (Glen Etive, 9th June 2016) Image © Peter Eeles |
Spean Bridge is found 8 miles or so north east of Fort William. The Chequered Skipper can be found here, albeit in low numbers, in suitable woodland bays and scrub found south of the River Spean. However, the density of individuals here is very low.
This species is, arguably, the most colourful of our skippers, the upperside exhibiting a mixture of browns and creams on a dark brown ground colour. The female has lighter upperside markings than the male as shown in Figure 40 and the underside is equally beautiful. Both sexes rest with their wings tightly shut during dull weather and at night. Butterflies may be active from 0700 on warm and sunny days, in sheltered areas that catch the early morning sun, until 1900, again on the warmest days (Ravenscroft, 1992).
Male Chequered Skippers are very easy to find at suitable sites since they are quite visible when protecting their territories. They will dart up at any passing object, including other species, an intrusion by another insect lasting only a few seconds, but an intrusion by another male resulting in a spiralling flight that could be quite prolonged which, according to Ravenscroft (1992), could result in them flying 20-30m outside of the occupant's territory before one of the males, usually the resident according to Ravenscroft (1994c), returns.
Ravenscroft (1992) also observed a male intercepting a passing object that was 4.1m from its perch and only rarely did insects get within 1m before being investigated. Males also have a habit of moving from perch to perch within their territory and Ravenscroft (1992) says that an encounter with another male results in the occupant making such flights more frequently. A lack of activity within a territory may result in the male moving to a new area.
The overall level of activity in the male requires a warm body, which is one of the main reasons that sheltered spots are favoured. According to Ravenscroft (1994c), mate location by males within their territories was most prevalent during the hottest parts of the day and, when not active within their territories, males can be found nectaring and basking.
If a virgin female is encountered by the male then she will lead him away in a short flight before landing, when the two mate, staying coupled for around 45 minutes based on a single observation made at Glasdrum NNR (Ravenscroft (1992) states between 40 minutes and 1 hour). A female that has already mated, however, will simply drop to the ground, ignoring the advances from her suitor by remaining motionless, the male constantly fluttering around her but eventually losing interest and flying off.
 |  |
| Figure 42 - Male Chequered Skipper (Glasdrum NNR, 28th May 2014) Image © Peter Eeles | Figure 43 - Male Chequered Skipper (Glasdrum NNR, 23rd May 2016) Image © Peter Eeles |
Female Chequered Skippers are by far the more difficult to find of the two sexes. This is almost certainly because, as mentioned in Ravenscroft (1992), females are more mobile than males, often moving out of the core areas once they have mated.
 |  |
| Figure 44 - Female Chequered Skipper (Glasdrum NNR, 22nd May 2016) Image © Peter Eeles | Figure 45 - Female Chequered Skipper (Glasdrum NNR, 23rd May 2016) Image © Peter Eeles |
Variation in this species is mainly with regard to the extent of the markings and a few aberrations were encountered during site visits.
When egg laying, a sight I have been privileged to witness only twice, the female alights on a grass blade, shimmies down it a short way, curls her abdomen under the leaf, then deposits a single white egg before flying off almost immediately, the entire event lasting no more than 15 seconds. Eggs are laid approximately half way up the leaf and never at the leaf tip or base. Most Purple Moor-grass plants will be home to a single larva, although there are exceptions of course, usually when two different females have laid on the same plant.
Ravenscroft (1992) says that females usually rest for long periods before laying each egg although, occasionally, he saw three or more laid in succession. He goes on to say that, on one occasion, a female laid an egg just 3 minutes after she had finishing mating.
Ravenscroft (1992) states that eggs are always laid on the upperside of a grass blade (the side without the mid-rib), while other authors state that the underside is used. My own observation is that eggs are almost always laid on the surface that is facing the ground (with just one exception and based on a very small sample) and I always assumed this to be the underside of the leaf. However, Ravenscroft shows that this is not so, with 42% of eggs laid on leaf uppersides facing up, and 58% laid on the uppersides facing down. My own photos would seem to confirm this observation and my conclusion is that there is some confusion over the meaning of "upperside" between authors.
Eggs are dome-shaped with a sunken micropyle, and a shell surface that exhibits a very subtle network pattern primarily made up of hexagons. They are approximately 1mm in diameter and are pure white when first laid, hatching after approximately 15 days (Ravenscroft (1992) gives a figure of 17-22 days and Frohawk (1892) gives a figure of 10 days when he reared this species in captivity from English stock).
A few days after being laid, the egg starts to colour up, becoming a very pale lemon-orange.
A couple of days before hatching, the black head of the larva becomes progressively more visible through the egg shell near the micropyle.
 |
| Figure 55 - Egg just prior to emergence (Glasdrum NNR, 11th June 2016) Image © Peter Eeles |
Even when an egg is known to be present on a tussock of Purple Moor-grass, it can still be difficult to locate. Given that eggs are laid under the leaf blade, I thought that an investment in a cheap inspection mirror (used to check the underside of cars) would be a good idea. However, this turned out to be ineffective since all you see is the silhouette of a grass blade against the sky. The better approach, which is also quicker than inspecting each blade in turn, is to gently fold back the tussock from various angles and inspect the leaves that way.
There are 5 instars in total, with the larva overwintering in the final instar. This number of instars, taken from Frohawk (1892) and which correlates with my own observations, does not agree with the 4 instars mentioned in Ravenscroft (1992) who considers the 3rd and 4th instars mentioned here to be one, although he does acknowledge the challenge of observing larvae that spend most of their time in a grass tube. The remainder of this discussion assumes 5 instars.
Only when fully grown in the last instar will the larva rest openly on a blade of grass. Up until that point, the larva seeks shelter in a tube that is constructed from one (early instars) or several (later instars) blades of Purple Moor-grass. The larva reverses out of this tube to eject frass. The role of the tube is almost certainly to avoid predation and Ravenscroft (1992) confirms that periods of mortality coincided with shelter changes (Ravenscroft (1996) cites spiders, harvestmen and sucking bugs as predators). Ravenscroft (1992) suggests that the tube may also provide a local microclimate that raises the temperature and is beneficial to the development of the larva, as well as controlling water loss.
The larva will feed both above and below the tube (this is true of all instars), resulting in characteristic feeding damage that allows the observer to more-easily locate larvae in the wild, especially those of the later instars when the tubes and associated feeding damage are more extensive. The feeding can sometimes be so extensive that only the midrib remains intact and the tube is long enough to just about conceal the larva and two examples from unknown instars are shown in Figures 56 and 57. Once this point has been reached then the larva will typically move to a new location and create a new tube, possibly on the same grass blade.
 |  |
| Figure 56 - Larval feeding damage (Glasdrum NNR, 1st September 2014) Image © Peter Eeles | Figure 57 - Larval feeding damage (Glasdrum NNR, 1st September 2014) Image © Peter Eeles |
One curious behaviour of the larva is that a gentle tap of its tube may cause it to quickly move forwards or backwards out of the tube, remaining motionless for some time before returning once the apparent danger has gone. I first discovered this when I accidentally knocked a tube while setting up my tripod to take a photo of a completely different tube. It was quite a memorable moment for me, since this is how I first came face-to-face with a final instar Chequered Skipper larva at Glasdrum in 2014, and is the single event that kick started this study.
This characteristic is especially useful when looking to illustrate or photograph the larvae, and was successfully exploited by Frohawk (Frohawk, 1892). Although often time-consuming, an alternative approach is to wait for the larva to commence feeding above or below its tube. It may even abandon its tube completely when feeding.
The larva eats the crown of the egg before emerging and goes on to eat the remainder of the egg shell, although a detectable "halo" can subsequently be seen at the egg site. The newly-emerged larva has a shiny black head with a black collar that is characteristic of many skipper larvae. The body is a light lemon colour with a small number of short fine hairs. The larva is approximately 2mm long when first emerged and this instar lasts approximately 15 days.
Once the eggshell has been eaten, the larva proceeds along the leaf (up or down), stopping when it finds the right width from which to form a protective tube. It may move to a nearby leaf before finding a suitable site.
 |  |
| Figure 60 - 1st instar larva taking its first meal (Glasdrum NNR, 13th June 2016) Image © Peter Eeles | Figure 61 - 1st instar larva (Glasdrum NNR, 13th June 2016) Image © Peter Eeles |
The tube is formed by using silk to bring the edges of the leaf together. The young larva will spend a considerable amount of time in this process, moving its head in successive side-to-side movements as it places silk on each leaf edge.
As the silk dries it contracts, resulting in the edges of the leaf being drawn together, thereby forming a tube in which the larva resides and from which it emerges to feed.
After the first moult the larva is nearly 6mm long (Frohawk, 1892), this instar lasting approximately 16 days (Frohawk (1892) gives a figure of 9 days). This instar is similar in appearance to the 1st instar larva in that it has a black, shiny head, a black collar, and the body is covered in a small number of short hairs. There are no distinctive markings.
After the second moult the larva is nearly 8mm long (Frohawk, 1892), this instar lasting approximately 10 days (Frohawk (1892) gives a figure of 13 days). The larva now exhibits several subtle longitudinal stripes, but the most distinctive change is the addition of a black mark on the last segment, in the shape of an hour glass.
After the third moult the larva is approximately 15mm long (Frohawk, 1892), this instar lasting approximately 20 days. The appearance is very similar to the 3rd instar, having the same markings, although the black mark on the final segment is particularly conspicuous. The larva is now of a size where a tube may be constructed from multiple leaves, rather than single leaf.
The larva initially takes on a very pale green colour after the 4th moult, turning a deeper green as it grows. However, the most significant change is that the head is no longer black, but a very pale green with a faint line separating the two lobes of the head. Also, the black collar and black mark on the final segment are absent. When fully grown, around the beginning of October, the larva is approximately 24mm long (Frohawk, 1892).
As the larva grows it becomes more free-living and can be found sitting out in the open on a blade that has a minimal silk pad that the larva clings to when inactive. It also wanders more than younger larvae that reside within a tube, and will more frequently be found on a neighbouring plant.
 |  |
| Figure 81 - 5th instar larva head capsule (Glasdrum NNR, 21st August 2015) Image © Peter Eeles | Figure 82 - 5th instar larva fully-grown (Glasdrum NNR, 18th September 2014) Image © Peter Eeles |
The feeding damage is also characteristic at this late stage, when the larva no longer creates tubes in which it resides, but simply eats a notch at the base of a blade before feeding above it. Ravenscroft (1992) suggests that this maintains higher nutrients in the grass above the notch for a few days.
 |
| Figure 83 - 5th instar larva showing characteristic feeding damage (Glasdrum NNR, 1st September 2014) Image © Peter Eeles |
In late October the larva prepares itself for hibernation (Ravenscroft (1994) says that some larvae were still active on 11th November in 1990). It does this by fixing the edges of two a more leaves together with silk, before sealing both ends. When the larva emerges in the spring, it will not feed further before pupating.
Given the Scottish weather, and the fact that the Purple Moor-grass dies back in the winter (it is a deciduous perennial, like all Molinea species), then the hibernacula do not always stay upright, and it was not unusual to discover that they had been blown horizontal with the leaves on which they were formed.
Around the end of March and early April, the larva emerges from hibernation, revealing an amazing evolutionary trick; what was a largely-green larva has transformed and is now a pale straw colour, perfectly matching the colour of a dead Purple Moor-grass leaf.
Unfortunately, the post-hibernation larvae are not only extremely well-camouflaged; they will also wander to adjacent plants. The analogy of looking for a needle in a haystack is quite appropriate when trying to find these masters of disguise, and I am extremely grateful to Mark Colvin who spent a couple of days helping me relocate larvae at Glasdrum in March 2016, as well as helping undertake a survey of all of the study sites for a week later that year.
The overwintering larva first shows itself while still in its hibernaculum, and it may remain in this state for a number of days before finally leaving its winter home. Once it does leave, the larva can sometimes be found resting on or under a grass blade, against which it is superbly camouflaged.
After a week or so, the larva constructs a flimsy "tent" from several dead grass blades. The larva then creates a silk pad at its tail end (which the pupal cremaster will eventually attach to) before creating a silken girdle to hold itself (and the subsequent pupa) in position.
The larva remains in this position for approximately a week before pupating, the 5th instar lasting approximately 230 days, with the larval stage lasting around 290 days in total.
The pupa is formed in early April, is approximately 16mm long (Frohawk, 1892), and remains in this state for just over 40 days before the adult emerges. A newly-formed pupa has a series of brown longitudinal stripes along its back and sides, which darken over the next few days, and has a noticeable "beak" on its head. The resulting pupa is most beautiful and well-camouflaged against the dead and withered grass, turning almost black as the time for emergence draws near.
Although I was never fortunate enough to see an adult emerge from the pupa (despite many hours waiting for the big event), I do believe that it is possible to sex the pupa based, primarily, on the width of the abdomen, where male pupae have a narrower abdomen than females.
As for overwintering larvae, pupae are also at the mercy of the plant to which they are attached, and are occasionally found lying almost horizontal if the dead blades have fallen over.
 |  |
| Figure 100 - Flattened blades of Purple Moor-grass (Glasdrum NNR, 13th April 2016) Image © Peter Eeles | Figure 101 - Male pupa lying almost horizontal (Glasdrum NNR, 14th April 2016) Image © Peter Eeles |
After analysing my own data with regard to one particular individual that was followed from egg to adult (see Table 1) in 2015-2016, I found a strong correlation with the durations given by Frohawk (1892) who had reared a single individual in 1891-1892. This was surprising since Frohawk reared English stock in captivity, whereas I monitored Scottish stock in the wild. However, it is difficult to draw conclusions from the data associated with a single individual. Unfortunately, other individuals that I had monitored either did not survive, were lost during the study or have incomplete data.
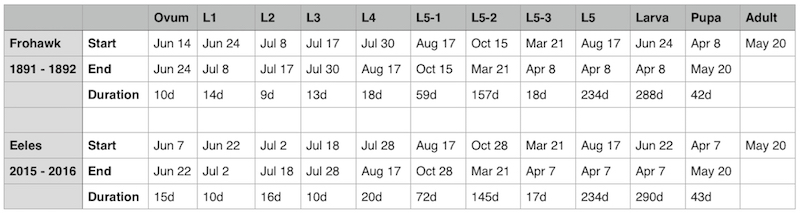 |
| Table 1 - Analysis of dates and duration of all stages and each larval instar |
Key To Larval Instars
My surprise at this correlation stems from assumptions I was making regarding both climate (I assumed colder and wetter in Scotland than in England) and flight times (I assumed that adults emerged later in Scotland, being considerably further north than the English colonies). My assumptions were wrong on both counts.
Firstly, the Scottish climate in this part of the world is not what you might think. This part of Scotland is certainly wet, with a high amount of rainfall (Newland (2011) showing that rainfall in July is potentially significant to the success of the species), but it is also warm with mild winters. The Gulf stream has a particular effect and, according to Ravenscroft (1992), the mean temperature is only 1 degree lower than lowland England with a small seasonal range of 8.5 degrees compared with 14 degrees for lowland England. Secondly, Collier (1986) states that the mean data suggests that the flight season was generally earlier in Scotland, which was also unexpected.
It is therefore, perhaps, not surprising that my own dates and those of Frohawk correlate more closely than one might initially expect. Given the data that Collier was examining (which included relatively-recent records up to the extinction of the butterfly in England in 1976) then it was felt that any effects from climate change could be ignored.
On the basis of all of my observations I was able to produce the detailed phenology chart shown in Figure 102. Filled circles represent actual observations and unfilled circles represent predictions based on those observations, while also recognising the work of Frohawk (1892) and Ravenscroft (1992).
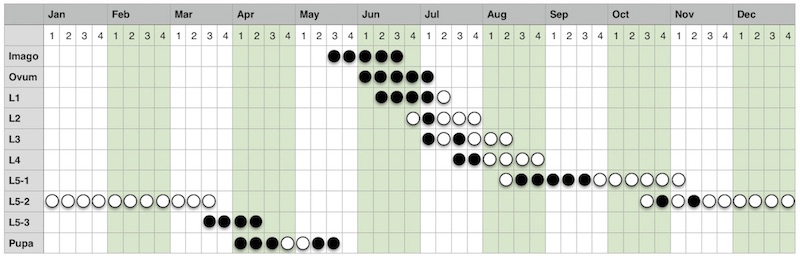 |
| Figure 102 - Detailed Chequered Skipper phenology chart Image © Peter Eeles |
This study has been one of the most challenging I have ever embarked upon, requiring a significant amount of time, effort and patience. It has also been the most rewarding. It has been a privilege to validate many of Ravenscroft's conclusions, as well as provide original observations on the immature stages in particular.
 |
| Figure 103 - Two rarities side by side: male Chequered Skipper and male Small Pearl-bordered Fritillary (Glasdrum NNR, 27th May 2014) Image © Peter Eeles |
Those interested in reading more should start with Ravenscroft (1992) which goes into much more detail, such as describing the methods he used, and adding implications for conservation which I have not included here. It will certainly provide inspiration for anyone looking to conduct a similar analysis on this species or any other species of Lepidoptera.
My particular thanks go to Mark Colvin for not only reviewing this article, but also for his company in the field while analysing study sites and tracking down immature stages.
I would also like to thank Neil Freeman who we fortuitously encountered at Glen Loy, and who was able to help us identify particular Chequered Skipper hotspots on the banks of the River Loy. His tip of using Avon's "Skin So Soft" product for combating midges was much appreciated!
Finally, I would like to thank Jane Turner and Chris Upson who I seem to meet at Glasdrum most years and, in 2016, were able to direct me to the exact plant on which they had seen a female Chequered Skipper lay an egg, saving me a considerable amount of time and also alerting me to a new area that was favoured by ovipositing females, thereby strengthening some of the conclusions made in this article.